
Search
The Renewable Energy site for Do-It-Yourselfers
Thoughts on Mik's Stored Solar Heat
Cooking Scheme
Insulation
Mik points out the need for an inexpensive insulator that will hold up to the
high temperatures that the concentrator produces -- about 1800F.
The Engineering Toolbox provides this list of high temperature insulators:
http://www.engineeringtoolbox.com/insulation-temperatures-d_922.html
Mineral Wool is listed at 1800F. I don't know if this is the same
material that Mik used and had problems with, or whether this was fiberglass, or
perhaps used binders with a lower temperature capability?
It looks like Perlite might also be a candidate:
http://www.engineeringtoolbox.com/perlite-insulation-k-values-d_1173.html
Another approach might be to make the first layer of insulation something
like fire bricks (as in the type used to make kilns), and then surround this
with something like Mineral Wool. The temperature drop across the fire
brick would allow the Mineral Wool to run at a lower temperature.
It does seem like much better insulation would be a big plus as right now Mik
is able to heat the steel bars up to around 1500F, but can't put them in the
insulated barrel until they cool to about 900F -- so there is some heat being
wasted here that might be used if a higher temperature insulation could be
found? This might make the fire brick approach worth trying?
Solar Heat Input
The reflector has an area of 12.6 sf or 1.2 sm. For full sun, this
would be about (12.6 sf)(300 BTU/hr-sf)(0.9 reflectivity) = 3402 BTU/hr
To heat up the steel rods from 70F up to 1500F takes about (37lb)(0.12 BTU/lb-F)(1500F
- 70F) = 6400 BTU.
Mik reports that it takes a few hours to get the rods up to the 1500F or so
-- this indicates to me that the concentrator is getting the heat into the rod
bundle fairly efficiently, as it would take nearly two hours of good sun to get
the rods up to 1500F at 100% efficiency.
Heat Storage Material
Sensible Storage
Mik's setup stores heat as "sensible" heat using steel rods. Sensible
meaning that the heat that the material absorbs or releases as it changes
temperature.
This is a excerpt from "Survey
of Thermal Storage for Parabolic Trough Power Plants", NREL
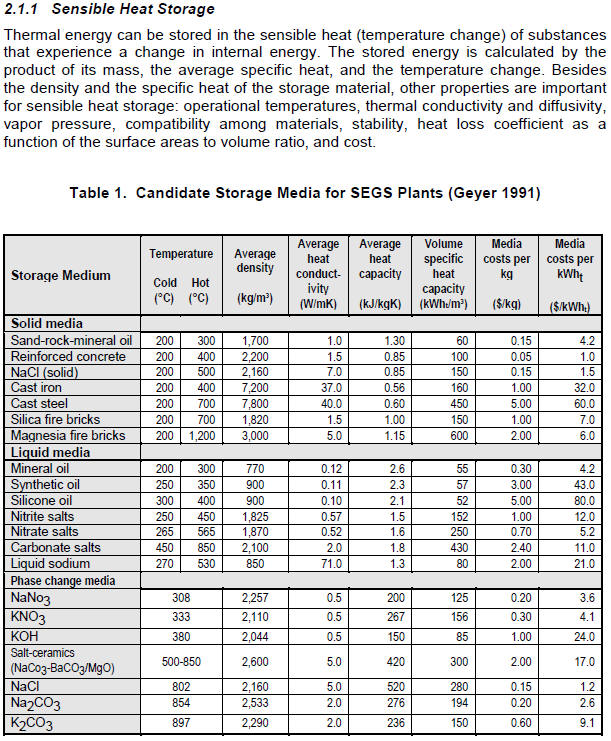
Looking over the list above of materials that might be used to store the
solar heat and also stand up to the high temperatures, the steel rods look about
as good as anything, and are readily available and cheap. But, there are a
few other materials that might be looked at -- e.g. Carbonate Salts? or Magnesia
fire bricks?
Using this sensible storage method, if the upper end of the usable
temperature range is is (say) 900F, and the lower end 250F, then each pound of
steel can store (0.12 BTU/lb-F)(1 lb)(900F - 250F) = 78 BTU. This
would be enough heat to warm one lb of water up 78F degrees if transferred with
100% efficiency.
The weight of Mik's steel rod bundle is about: (100
rods)(0.4*0.4in^2)(8 in)(0.285 lb/in^3) = 37 lbs.
So, the using the 250F to 900F range, the total storage is about (78 BTU/lb)(37lbs)
= 2900 BTU -- some of this would be lost with the less than 100% efficient
transfer to the food, but, it only takes about 1000 BTU to raise the temperature
of a gallon of stew from 70F up to 200F -- so, as Mik has already shown, that
there is enough heat here to do some useful cooking.
Latent or Phase Change Storage
Latent heat storage takes advantage of the fact that for most materials, it
takes a lot of energy to change the material from a solid to a liquid, and this
heat can be recovered at a later time. One advantage of this method is
that the temperature remains constant during the time that the material is being
converted from a liquid to a solid -- its basically a constant temperature heat
source. The downside would be that it would take a good
vessel to hold the molten phase change material and the safety issues for a
molten material seem more problematic.
Looking at the "Phase change media" section of the table above, it looks like
there are a couple salts that might be used (NaCl (table salt) or the "Salt
Ceramics").
Using the numbers in the table above for the best phase change material
(Salt-ceramics), it would take (2900 BTU)/(16.8 BTU/in^3) = 172 cubic inches of
he salt -- this is compared to 128 cubic inches of the steel that Mik used.
So, volume wise, its not as efficient as the steel . It appears that the
only real advantage would be the constant temperature as it releases heat.
The Cooking from Stored Heat
It seems like an insulating cooking pot would be worth using when possible --
this might make the stored heat last much longer.
Thermal cookware blog...
Hot box cooking... This might allow cooking two things at once from
the same stored heat source.
Gary
January 7, 2012

